![]() Originally identified by Reil (1809) and subsequently named by Burdach (1819), the arcuate fasciculus is a white-matter, neural pathway that intersects with both the lateral temporal cortex and frontal cortex via a “dorsal projection that arches around the Sylvain fissure.” (Rilling et al., 2008, pg. 426). Classical hypotheses saw this pathway as a critical component in connecting two centres of language: Broca’s area (speech production) and Wernicke’s area (speech comprehension) (Catani and Mesulam, 2008).
Originally identified by Reil (1809) and subsequently named by Burdach (1819), the arcuate fasciculus is a white-matter, neural pathway that intersects with both the lateral temporal cortex and frontal cortex via a “dorsal projection that arches around the Sylvain fissure.” (Rilling et al., 2008, pg. 426). Classical hypotheses saw this pathway as a critical component in connecting two centres of language: Broca’s area (speech production) and Wernicke’s area (speech comprehension) (Catani and Mesulam, 2008).
Much of these assumptions were based on a tentative relationship between language-impairment and damaged portions of the brain. Notably, damage to the arcuate fasciculus is implicated in a syndrome known as conduction aphasia, where an individual has difficulty in speech repetition. Often characterised by errors in spontaneous speech, an individual with conduction aphasia will be fully aware of their mistake, retaining well-preserved auditory comprehension and speech production while also being syntactically and grammatically correct (ibid).
In addition, these lesions were in the left-hemisphere (LH), leading to further speculations on a left-lateralisation for language processing. Though as we now know: language is far more distributed, and not just confined to the left-hemisphere (LH). In fact, the right-hemisphere (RH) has been shown to process prosody, discourse comprehension, connotative meaning and many other language phenomena (cf. Bookheimer, 2002). Furthermore, recent studies (cf. Hickok and Poeppel, 2004; Dronkers et al., 2007) demonstrate the arcuate fasciculus may not implicitly involved in conduction aphasia.
Despite its diminished role in language, the arcuate fasciculus is part of a renaissance in neuroscientific investigation, largely due to recent advances in brain imaging techniques. With these new methods of analysis come new hypotheses for functional aspects of brain regions and their neural networks. In particular, the dual stream model for speech and language processing (Hickok and Poeppel, 2004) has become a key in discerning the arcuate fasciculus’ role (Glasser and Rilling, 2008; Saur et al., 2008). With this in mind, the essay will now discuss and review these accounts of the arcuate fasciculus, and attempt to place it within current accounts of speech processing.
2. The anatomy, function and evolution of the arcuate fasiculus
2.1 The flaws in classical accounts of anatomy and function of the arcuate fasciculus
Prior efforts in discerning the anatomical aspects of arcuate fasciculus[1] were largely based on observations by Constantin Von Monakow, Joseph Jules Dejerine and, later on, Norman Geschwin (cf. Catani and Mesulam, 2008). Much of these earlier studies relied on post-mortems in humans, employing relatively crude methods, such as blunt dissections and myelin stain techniques (ibid).
These methods did not reveal much in-depth information concerning the arcuate’s specific anatomical terminations, just a basic outline of its pathway – stretching from the temporal lobe to the frontal lobe. This putative anatomy was used as the basis for hypothesising the arcuate’s function: a language substrate that connected speech comprehension (Wernicke’s area) with speech production (Broca’s area). More importantly, confirmation of this hypothesis relied on accounts that disconnecting the arcuate would result in conduction aphasia (ibid).
Specifically, original accounts of conduction aphasia distinguished between two groups:“[…] the Broca-like syndrome in which the deficit in repetition is accompanied by a relative impairment in fluency and the Wernicke-like syndrome in which the deficit in repetition is accompanied by a relative impairment in comprehension. One explanation for this dichotomy is that more anterior lesions encroach on Broca’s area, whereas more posterior lesions encroach on Wernicke’s area” (Catani et al., 2004, pg. 13).
These disconnectionist approaches would later be challenged by a range of studies (including: Damasio and Damasio, 1980; Naeser et al., 1982), showing the classical arcuate cannot explain cases where Broca-like and Wernicke-like conduction aphasias emerge from subcortical lesions. Subsequent studies (Hickok et al., 2000; Dronkers et al., 2007) also argue conduction aphasia is not even associated with the arcuate. This is demonstrated in several ways, including: arcuate lesions not inducing conduction aphasia (Selnes et al., 2002); left auditory cortical lesions causing conduction aphasia (Graves et al., 2008); and, cortical stimulation eliciting conduction aphasia-like symptoms (ibid).
2.2 Arcuate Fasciculus as a direct phonetic pathway
More recently, diffusion tensor imaging (DTI) tractography, a method of reconstructing white matter pathways by tracing the diffusion of water, reveals a more complex picture of the arcuate fasciculus (Catani and Mesulam, 2008).
Here, one group of studies (Catani et al., 2004; Parker et al., 2005; Powell et al., 2006) not only confirm some findings from the classical accounts, which connects the temporal lobe with the frontal lobe, they also demonstrate additional indirect pathways outside of the typical perisylvian connectivity (see figure 1). Also, there appears to be a far more extensive branching of the arcuate’s cortical terminations “[…] beyond the classical limits of Broca’s and Wernicke’s areas to include part of the middle and precentral gyrus and the posterior middle temporal gyrus, respectively.” (Catani and Mesulam, 2008, pg. 957).
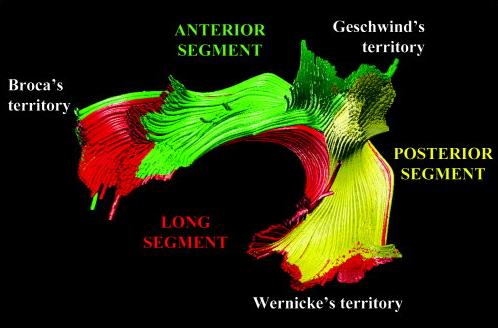
Another reaffirmation is the apparent hemispheric asymmetry in the arcuate fasciculus. Again using DTI, Catani et al. (2007) find differences in connection patterns between the right and left hemispheres, showing a leftward lateralisation in ~80% of tested subjects. Despite language functions showing a greater degree of hemispheric distribution than originally thought (Bookheimer, 2002), left-lateralised asymmetry is still considered to be a key aspect of language processing (Catani and Mesulam, 2008).
Makris et al. (2005) observe that the left-lateralised arcuate runs alongside neuronal pathways in the superior longitudinal fasciculus (SLF). They claim the SLF can be split in four distinct components, one of which being the arcuate, and claim (on the basis of previous work by Petrides and Pandya, 2002) these fibre pathways are bidirectional. They also present a detailed anatomical description of the arcuate, showing that neurons branching out from the caudal superior temporal gyrus (which includes wernicke’s area) and the superior temporal sulcus, curving around the Sylvian fissure (caudal portion), which then: “[…] runs along with the fibers of SLF II […] AF [arcuate fasciculus] fibers continue into the frontal lobe and terminate predominantly in the dorsal part of area 8 and in area 46. AF becomes distinct mainly at the parietal opercular level.” (ibid, pg. 863).
These accounts place the classical, left-lateralised arcuate as a direct phonetic pathway, where phonologically-rooted functions are sent directly to the frontal cortex. As Catani and Mesulam (2008) explain, in discovering two additional pathways (indirect) that connect to Geschwind’s territory (corresponding to areas BA 39 and 40), the expanded arcuate also assumes a role in processing semantic information. Although, attributing semantic processing to BA 40 may not necessarily hold true, and might just be limited to BA 39 (Price, 2000). Glasser and Rilling (2008) raise issue with this, also adding that BA 40 is “[…] more likely to be involved in phonetic working memory than semantic processing, and semantic processing is found far more often in the temporal lobe.” (pg. 9). Lastly, Johnson-Frey (2008) found that grasping actions and tool use planning are represented in the left inferior parietal lobe (BA 39), leading to speculations that Geschwind’s territory might primarily be involved in complex tool use (Glasser and Rilling, 2008).
However, these anatomical and functional accounts are far from unequivocal. Another group of papers (Schmahmann et al., 2007; Saur et al., 2008; Frey et al., 2008) also place the arcuate as a direct phonetic pathway; however, they find no evidence of arcuate pathway terminations in BA 45. The basis of this assertion rests on comparative studies between humans and macaques, arguing that anatomical analysis suggests it is the extreme capsule (EmC) and the MdLF are involved in language, not the arcuate (Schmahmann et al., 2007). Instead, the arcuate, SLF, MLF and ILF (inferior longitudinal fasciculus) form a composite fibre bundle that “[…] is mainly restricted to sensory-motor mapping of sound to articulation.” (Saur et al., 2008, pg. 18035).
2.3 Arcuate fasciculus as a phonological, lexical-semantic and prosodic processor
Running contrary to the notion of the arcuate as a direct phonetic pathway are Glasser and Rilling (2008), who hypothesise it is comprised of different routes and different functions. Specifically, they posit two pathways: one terminating in the posterior superior temporal gyrus (STG) and the other in the middle temporal gyrus (MTG), with these neural pathways involving phonetic and lexical-semantic processing, respectively. Interestingly, they also implicate the right-hemispheric arcuate in phonological processing (bilateral activation) and during prosodic processing (right-lateralised activation). Both of these findings being confirmatory of a wider distributed processing of language, particularly in the right-lateralisation of prosodic processing (Ethofer et al., 2006).
In a related study, Rilling et al. (2008) further delineate the arcuate, showing specific termination points, with the superior, middle and inferior temporal gyri making up the temporal projection, whilst the frontal projection consists of the ventral premotor cortex (BA 6), pars opercularis (BA 44), pars triangularis (BA 45) and the middle frontal gyrus (BA 9). Furthermore, they offer a comparative account between the arcuate connectivity in humans, chimpanzees and macaques.
In investigating the respective pathways of all three primates, Rilling et al. show how significant differences in these lineages are suggestive of gradual modifications to cortical terminations during human evolution (see figure two). As such, chimpanzees display more structural similarity to humans in their pathway trajectories, with extensive terminations in the frontal region; however, terminations in the middle and frontal gyri are less pronounced. Being of a phylogenetically older lineage, macaques lack any temporal lobe terminations.
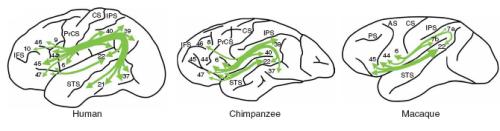
When combined with data from two additional fibre tracts (SLF and the extreme capsule), each predicted not to show any significant differences across all three of the primates, the study reveals a very strong case for the arcuate having being subjected to selective pressure on the human lineage (ibid) – somewhat countering the claims of Schmahmann et al. (2007). Even if we accept the possibility of selection, the arcuate might not have been selected because of its apparent role in language. As the authors note, there is the possibility for aspects of the arcuate being involved in other cognitively demanding behaviours, such as tool use (cf. Johnson-Frey, 2008). However, “[…] the correspondence between the structures modified in human evolution identified in this study and structures known to be involved in language function is notable.” (Rilling et al., 2008, pg. 428).
[1] Actually, the study breaks the numbers down as follows: extreme degree of leftward lateralisation ~60%; mild leftward lateralisation ~20%; and bilateral symmetry ~20%.
Main References
CATANI, M., & MESULAM, M. (2008). The arcuate fasciculus and the disconnection theme in language and aphasia: History and current state Cortex, 44 (8), 953-961 DOI: 10.1016/j.cortex.2008.04.002
Glasser, M., & Rilling, J. (2008). DTI Tractography of the Human Brain’s Language Pathways Cerebral Cortex, 18 (11), 2471-2482 DOI: 10.1093/cercor/bhn011